Simulating Osmosis with Orbeez Water Beads

Introduction
Over the years, I have tried numerous “osmosis” labs. I’ve used gummy bears, potatoes, grapes, and eggs to explain tonicity and its applications to cellular processes, but these labs were a little underwhelming. This year, my goal was to create a lab that was not only meaningful to the students, but applicable to real life experiences. Today, on the blog, I have a fun and educational lab that will check all the boxes!
Be sure to scroll to the bottom of the post to grab a FREE copy of this lab.
Goal
In this lab, your students will use water beads to simulate the process of osmosis in red blood cells. They will use the data collected from this simulation to make connections to real-life: specifically the effect of differing solute concentrations on human red blood cells.

To prepare for this lab, you will need the following materials:
- 4 large containers (I use 1000 mL beakers)
- Labels
- NaCl (approximately 118 grams)
- Distilled Water (4000 mL)
- Water beads (to replicate red blood cells, I like to use the red beads) (3 per lab group)
Directions:
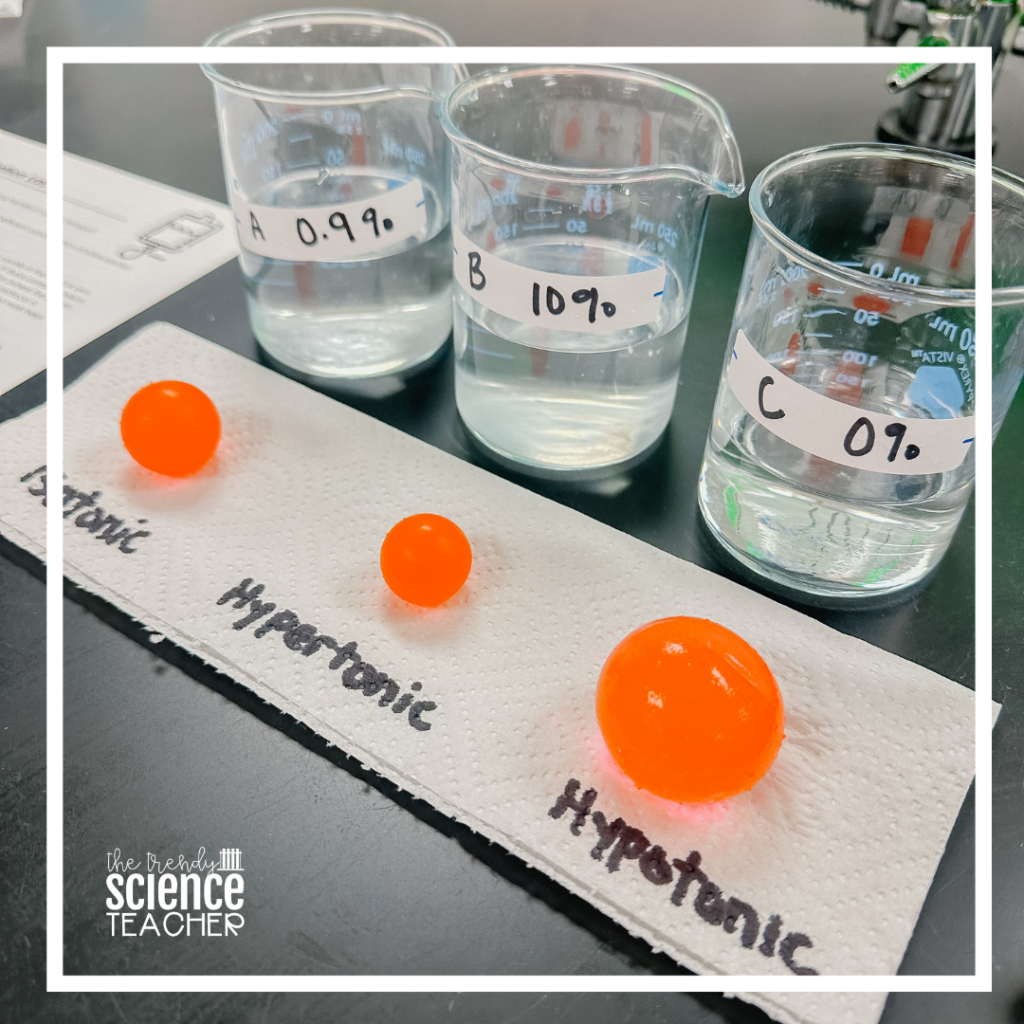
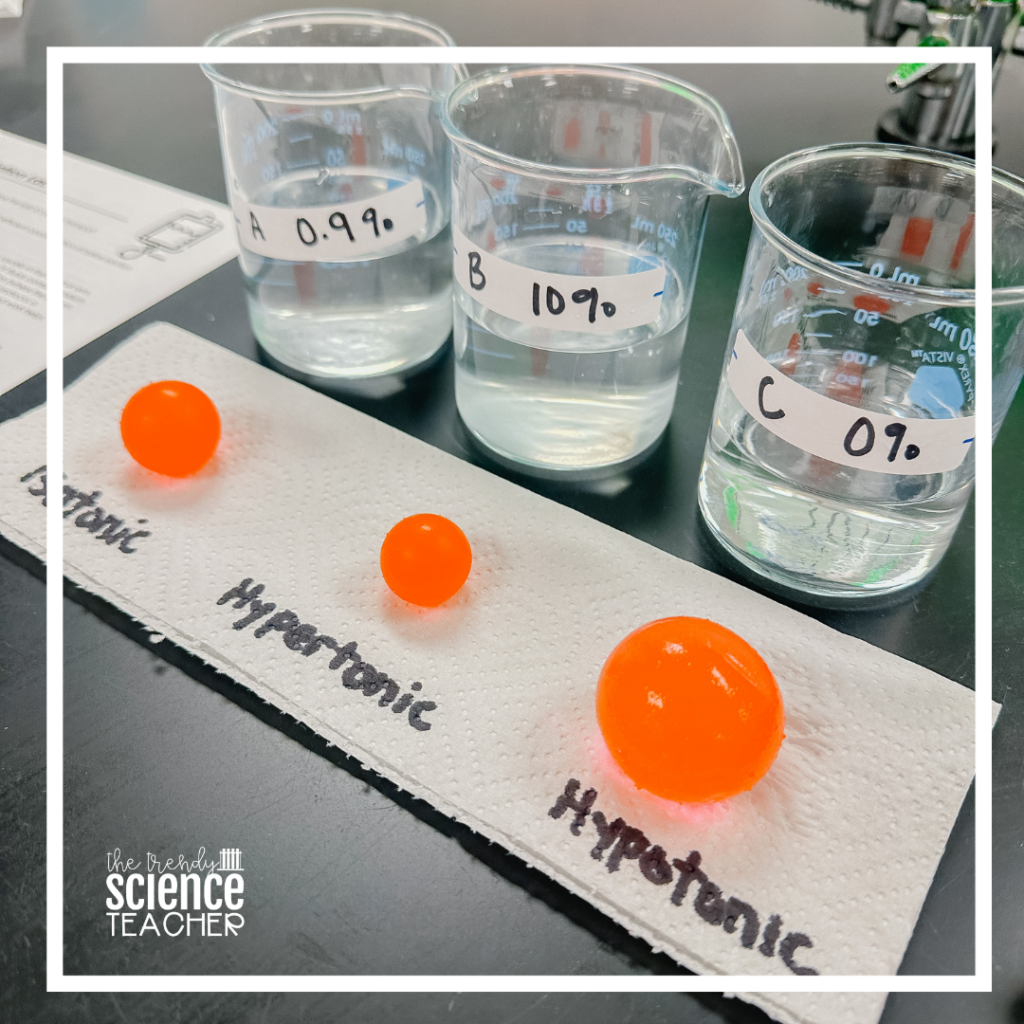
1. Label your containers A, B, C, & soaking solution. Container A will hold a 0.9% saline solution, container B will hold a 10% saline solution, and container C will hold a 0% saline solution. The soaking solution will be used to soak your water beads.
2. Prepare the three solutions (recipe below) for your students. The recipe below will provide enough solution for a class of 7 lab groups.
Solution A: 0.9% saline– Dissolve 9 g NaCl into 1000 mL of distilled water.
Solution B: 10% saline– Dissolve 100 g NaCl into 1000 mL of distilled water.
Solution C: 0% saline– Pour 1000 mL of distilled water into the container.
Soaking Solution: 0.9% saline– Dissolve 9 g NaCl into 1000 mL of distilled water.

3. Place water beads into the soaking solution. You will need to soak 3 water beads for each lab group that you have.

4. Allow the water beads to soak overnight.


For the lab:
Each lab group will need 150 mL of solution A, 150 mL of solution B, and 150 mL of solution C. (Note: I place these solutions on a cart and have lab groups come and pour them from my containers.)

Each group will also need:
3 – 200 mL beakers, labels, soaked 0.9% saline water beads, paper towels, a digital balance, a copy of the student lab sheet, a weigh boat, labels, and a marker/pencil
Students will use the water beads to represent the red blood cell of a healthy human (0.9% NaCl). They will place a red blood cell in 3 different solute concentrations to determine the solute’s effect on the red blood cell.
This lab will need to take place over the span of two days. I have not had success with allowing the solutions to set over the weekend, so I recommend planning for two consecutive days.
Prior to the lab:
Your students should have a basic idea of how osmosis works before completing this lab activity. I usually teach passive transport and spend a couple of days working through osmosis practice problems prior to this lab.
FAQs:
What do I do after the lab on Day 1 and 2?
The set up for Day 1 of the lab usually only takes about 15-20 minutes and Day 2 of the lab takes roughly 20 minutes. This leaves me with a little extra time on both days. I like to have an assignment for my students to work on after they finish their lab procedures for day 1 & 2. I assign my Cell Homeostasis: Osmosis Virtual Lab. It compliments this lab perfectly and provides extra practice over homeostasis/osmosis.
Can I use tap water?
No….tap water will not yield adequate results for the isotonic and hypertonic solutions. Distilled water is very important for this lab.
Can I use regular table salt?
Some people may argue that using table salt (without iodine) will yield adequate results; however, that has not been the case for me. This lab works best using lab grade Sodium chloride.
I hope that you have found today’s post helpful. If you are looking for a way to reinforce the process of osmosis during your cell transport unit, then be sure to check out this lab.

Share it:
- Read more about: BIOLOGY




